|
Plant–environment microscopy tracks interactions of Bacillus subtilis with plant roots across the entire rhizosphere
Friday, 2021/12/03 | 08:47:21
|
|
Yangminghao Liu, Daniel Patko, Ilonka Engelhardt, Timothy S. George, Nicola R. Stanley-Wall, Vincent Ladmiral, Bruno Ameduri, Tim J. Daniell, Nicola Holden, Michael P. MacDonald, and Lionel X. Dupuy
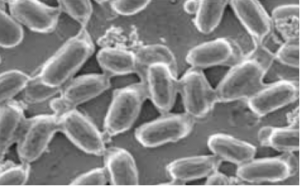
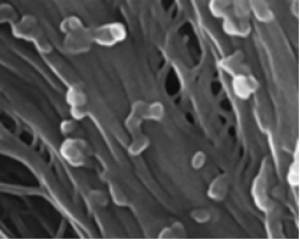
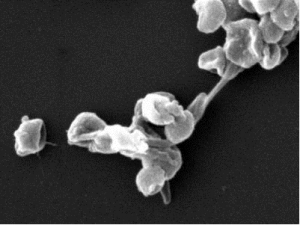
PNAS November 30, 2021 118 (48) e2109176118 SignificanceThe lack of suitable approaches for studying root–microbe interactions, live and in situ, has severely limited our ability to understand the rhizosphere. In this study, we overcome this major limitation with an imaging system that combines transparent soils with cutting edge light sheet microscopy. The study revealed that the root cap is a point of first contact for microbes before establishment and reveals how the pore structure influences the patterns of interactions between the microbe and the plant. With the combined use of light sheet microscopy and transparent soils, we shed light on previously unseen interaction phenomena and accelerate the understanding of how rhizospheres are formed. AbstractOur understanding of plant–microbe interactions in soil is limited by the difficulty of observing processes at the microscopic scale throughout plants’ large volume of influence. Here, we present the development of three-dimensional live microscopy for resolving plant–microbe interactions across the environment of an entire seedling growing in a transparent soil in tailor-made mesocosms, maintaining physical conditions for the culture of both plants and microorganisms. A tailor-made, dual-illumination light sheet system acquired photons scattered from the plant while fluorescence emissions were simultaneously captured from transparent soil particles and labeled microorganisms, allowing the generation of quantitative data on samples ∼3,600 mm3 in size, with as good as 5 µm resolution at a rate of up to one scan every 30 min. The system tracked the movement of Bacillus subtilis populations in the rhizosphere of lettuce plants in real time, revealing previously unseen patterns of activity. Motile bacteria favored small pore spaces over the surface of soil particles, colonizing the root in a pulsatile manner. Migrations appeared to be directed toward the root cap, the point of “first contact,” before the subsequent colonization of mature epidermis cells. Our findings show that microscopes dedicated to live environmental studies present an invaluable tool to understand plant–microbe interactions.
See: https://www.pnas.org/content/118/48/e2109176118
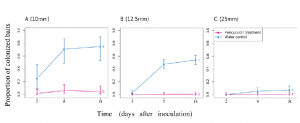
Fig. 5. Dynamics of the colonization of lettuce roots by B. subtilis across the whole dataset. (A) The colonization is marked by an increase in bacterial cell density in the soil further away from the root (red) within the first 26 h following inoculation, after which a maximum is reached between 30 and 34 h following inoculation. Bacterial cell density increases more persistently closer to the root surface (blue) until 30 to 34 h following inoculation and does not really reach a steady state in the analyzed time frame. Even though the bacteria total quantity seemed to reach a maximum after 26 h (green), variations in cell density along the root persisted. This indicates that migration may play a role in the later stages of the colonization of the root. (B) Intense activity at the root tip may precede colonization near or on the root surface. (Left) Example of the diagram of the colonization kinematics shows how cell density changes both with time and as a function of the position along one root. The diagram shows that densification of bacterial cell population is discrete (here, two pulses 7 h apart are recorded at ∼1 mm from the tip) and likely results from the attachment of bacteria on nongrowing tissue, since diagonal patterns indicate the constant increase in the distance from the root tip. (Middle) Overall, the distribution of bacterial cell density along the root (solid blue line) confirms that bacterial cell density concentrates in the basal region of the root (>1 mm from the root tip). (Right) On the contrary, the most intense temporal variations in bacterial cell density are observed near the root tip (dashed blue line). The activity of bacterial cells in the bulk soil (red) confirmed bacterial activity at the tip of the root is enhanced, with both the density in the bulk soil (solid red line) and the variance (dashed red line) showing a maximum in the region near the root tip. Data shown as mean ± SE, n = 6. |
|
|
|
[ Other News ]___________________________________________________
|


 Curently online :
Curently online :
 Total visitors :
Total visitors :
(165).png)