|
The cAMP signaling module regulates sperm motility in the liverwort Marchantia polymorpha
Friday, 2024/04/19 | 08:48:22
|
|
Chiaki Yamamoto, Fumio Takahashi, Noriyuki Suetsugu, Kazumasa Yamada, Shinya Yoshikawa, Takayuki Kohchi, and Masahiro Kasahara. PNAS; April 9, 2024; 121 (16) e2322211121 https://doi.org/10.1073/pnas.2322211121
Figure: The liverwort Marchantia polymorpha Significance3′,5′-cyclic monophosphate (cAMP) is well known to act as a second messenger in a wide range of organisms from prokaryotes to eukaryotes. Conversely, the function of cAMP signaling in land plants has long been disputed. Herein, we report that CAPE, a dual-functioning enzyme containing adenylyl cyclase and cAMP phosphodiesterase, and the regulatory subunits of cAMP-dependent protein kinase control sperm motility in the basal land plant Marchantia polymorpha. This study demonstrates that cAMP signaling plays a central role in sexual reproduction via motile sperm in land plants. Our findings, revealed using the reverse genetics approach, indicate that M. polymorpha would be a suitable model for the study of sperm flagellar motility. AbstractAdenosine 3′,5′-cyclic monophosphate (cAMP) is a universal signaling molecule that acts as a second messenger in various organisms. It is well established that cAMP plays essential roles across the tree of life, although the function of cAMP in land plants has long been debated. We previously identified the enzyme with both adenylyl cyclase (AC) and cAMP phosphodiesterase (PDE) activity as the cAMP-synthesis/hydrolysis enzyme COMBINED AC with PDE (CAPE) in the liverwort Marchantia polymorpha. CAPE is conserved in streptophytes that reproduce with motile sperm; however, the precise function of CAPE is not yet known. In this study, we demonstrate that the loss of function of CAPE in M. polymorpha led to male infertility due to impaired sperm flagellar motility. We also found that two genes encoding the regulatory subunits of cAMP-dependent protein kinase (PKA-R) were also involved in sperm motility. Based on these findings, it is evident that CAPE and PKA-Rs act as a cAMP signaling module that regulates sperm motility in M. polymorpha. Therefore, our results have shed light on the function of cAMP signaling and sperm motility regulators in land plants. This study suggests that cAMP signaling plays a common role in plant and animal sperm motility.
See https://www.pnas.org/doi/10.1073/pnas.2322211121
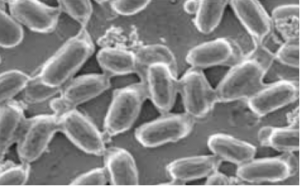
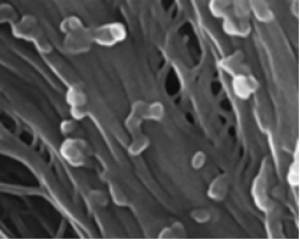
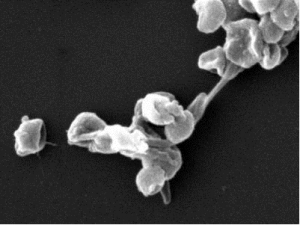
Figure 1: Spatiotemporal profile of CAPE gene expression and reverse genetic analysis of CAPE in M. polymorpha (Mp). (A) The GUS-stained images at each development stage of antheridiophore. Antheridiophore of stages 1 to 5 are shown from Left to Right, respectively. (B) The antheridia from stage 3 antheridiophore are shown. (C) Schematic representation of the MpCAPE gene and MpCAPE protein. Exons and introns are shown by boxes and lines, respectively. The positions of the gRNAs used to generate Mpcapege lines are shown. gRNA1 was used to generate Mpcape-1ge and Mpcape-2ge, gRNA2 was used to generate Mpcape-3ge and Mpcape-4ge. (D) Representative images of sperm in the WT and Mpcapege lines. The cell bodies (asterisks), anterior flagella (arrowheads), and posterior flagella (arrows) of sperm were observed using SEM. (E) Length of cell bodies, anterior flagella, and posterior flagella in the WT and Mpcapege lines. The numbers of sperm bodies, anterior flagella, and posterior flagella were n = 11, 8, and 9 (WT), n = 15, 10, and 11 (Mpcape-1ge), n = 11, 7, and 8 (Mpcape-2ge), n = 14, 12, and 12 (Mpcape-3ge), and n = 11, 10, and 10 (Mpcape-4ge) for the analysis of each length. Significant differences from WT were determined by Dunnett’s test: *P < 0.05. (F) Total cAMP levels of thalli; th, antheridiophores; an, and archegoniophores; ar, in WT and male Mpcapege mutant lines. *P < 0.05 by Dunnett’s test for multiple comparisons. n = 3 in each sample. (G) Total cAMP levels of sperm in WT and Mpcapege lines. *P < 0.05 by Dunnett’s test for multiple comparisons. Data are obtained from three biological replicates (n = 4 sperm suspensions for each biological replicate). (Scale bars, 1 mm (A), 100 μm (B), and 10 μm (D).)
|
|
|
|
[ Other News ]___________________________________________________
|


 Curently online :
Curently online :
 Total visitors :
Total visitors :
(236).png)
(328).png)