|
Regulation of MIR165/166 by class II and class III homeodomain leucine zipper proteins establishes leaf polarity
Monday, 2016/10/24 | 07:59:31
|
|
Paz Merelo, Hathi Ram, Monica Pia Caggiano, Carolyn Ohno, Felix Ott, Daniel Straub, Moritz Graeff, Seok Keun Cho, Seong Wook Yang, Stephan Wenkel, and Marcus G. Heisler SignificanceLeaves, being the prime photosynthetic organ of plants, are critical in many ways to our current biosphere. A defining characteristic, which also optimizes their function, is their flat shape that depends on the correct patterning of their upper and lower tissues during development. Here, we show that the correct patterning of upper and lower leaf tissues depends on two types of transcription factors (class II and class III homeodomain leucine zipper (HD-ZIPs) that act together to repress a set of miRNAs (MIR165/166), which in turn, represses the activity of these transcription factors (class III HD-ZIPs). This three-way interaction maintains the balance of tissue identities during growth, leading to the formation of a flat leaf. AbstractA defining feature of plant leaves is their flattened shape. This shape depends on an antagonism between the genes that specify adaxial (top) and abaxial (bottom) tissue identity; however, the molecular nature of this antagonism remains poorly understood. Class III homeodomain leucine zipper (HD-ZIP) transcription factors are key mediators in the regulation of adaxial–abaxial patterning. Their expression is restricted adaxially during early development by the abaxially expressed microRNA (MIR)165/166, yet the mechanism that restricts MIR165/166 expression to abaxial leaf tissues remains unknown. Here, we show that class III and class II HD-ZIP proteins act together to repress MIR165/166 via a conserved cis-element in their promoters. Organ morphology and tissue patterning in plants, therefore, depend on a bidirectional repressive circuit involving a set of miRNAs and its targets.
See: http://www.pnas.org/content/113/42/11973.abstract.html?etoc PNAS October 18 2016; vol.113; no.42: 11973–11978
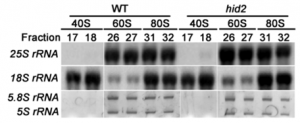
Fig. 1. HAT3 and ATHB4 are required for repressing MIR165/166 expression. (A–D) Expression of pREV::REV-2xYPET (red) in combination with the auxin efflux carrier PIN-FORMED1 (30, 31) fused to GFP (pPIN::PIN1-GFP) (blue) in the shoot apex of 4-d-old control (A and C) and hat3 athb4 plants (B and D). pPIN::PIN1-GFP is used here to outline the tissue. (C and D) Cross-sections of the same leaf primordia shown in A and B, respectively. (E–H) Expression of pMIR165a::mTagBFP-ER (green) in the shoot apex of 4-d-old control (E and G) and hat3 athb4 plants (F and H). (G and H) Cross-sections of the same leaf primordia shown in E and F, respectively. (I–L) Expression of a miR165/166 sensitive biosensor (white) containing the miRNA target sequence from REV fused to the UV-photoconvertible fluorescent protein mEos2FP (pUBQ10::REV-mEos2FP-ER) in the shoot apex of 4-d-old control (I and K) and hat3 athb4 plants (J and L). The sensitive biosensor is inactivated in cells where miR165/166 are active. (K and L) Cross-sections of the same leaf primordia shown in I and J, respectively. Chlorophyll autofluorescence: red (E–L). (Scale bars, 50 μm.) Ad, adaxial side; Ab, abaxial side. (M) Small RNA Northern blot showing expression levels of miR165/166 and U6 snRNA in Col-0 WT (lane 1), p35S::miR165a (lane 2), hat1 hat2 (lane 3), and hat3 athb4 plants (lane 4). |
|
|
|
[ Other News ]___________________________________________________
|


 Curently online :
Curently online :
 Total visitors :
Total visitors :
(39).png)