|
CRISPR/Cas9-based genome editing and functional analysis of SlHyPRP1 and SlDEA1 genes of Solanum lycopersicum L. in imparting genetic tolerance to multiple stress factors
Thursday, 2024/01/25 | 08:11:45
|
|
Banashree Saikia, Remya S, Johni Debbarma, Jitendra Maharana, G Narahari Sastry, C Chikkaputtaiah (figure) Frontiers in Plant Science; Volume 15 - 2024 | doi: 10.3389/fpls.2024.1304381
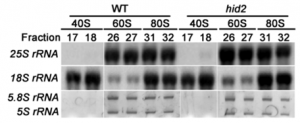
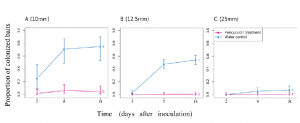
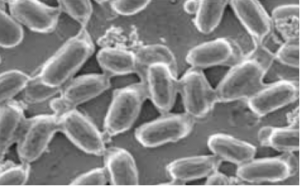
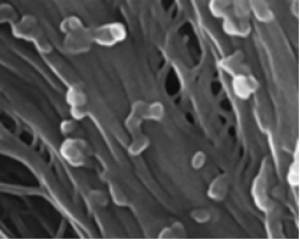
CRISPR/Cas is a breakthrough genome editing system because of its precision, target specificity and efficiency. As a speed breeding system, it is more robust than the conventional breeding and biotechnological approaches for qualitative and quantitative trait improvement. Tomato (Solanum lycopersicum L.) is an economically important crop but its yield and productivity have been severely impacted due to different abiotic and biotic stresses. Recently identified SlHyPRP1 and SlDEA1 are two potential negative regulatory genes in response to different abiotic (drought, salinity) and biotic stress (bacterial leaf spot, bacterial wilt) conditions in S. lycopersicum L. The present study aimed to evaluate the drought, salinity, bacterial leaf spot and bacterial wilt tolerance response in S. lycopersicum L. through CRISPR/Cas9 genome editing of SlHyPRP1 and SlDEA1 and their functional analysis. The transient single and dual-gene SlHyPRP1 and SlDEA1 CRISPR-edited plants were phenotypically better responsive to multiple stress factors taken under the study. CRISPR-edited SlHyPRP1 and SlDEA1 plants showed a higher level of chlorophyll and proline content compared to WT plants under abiotic stress conditions. ROS accumulation and the cell death count per total area of leaves and roots under biotic stress were less in CRISPR-edited SlHyPRP1 and SlDEA1 plants compared to WT plants. The study reveals that the combined loss-of-function of SlHyPRP1 along with SlDEA1 is essential for imparting significant multi-stress tolerance (drought, salinity, bacterial leaf spot and bacterial wilt) in S. lycopersicum L. The main feature of the study is the detailed genetic characterization of SlDEA1, a poorly studied 8CM family gene in multi-stress tolerance through CRISPR/Cas9 gene editing system. The study revealed the key negative regulatory role of SlDEA1 function together as an anchor gene with SlHyPRP1 in imparting multi-stress tolerance in S. lycopersicum L. It was interesting that the present study also showed that transient CRISPR/Cas9 editing events of SlHyPRP1 and SlDEA1 genes were successfully replicated in stably generated parent genome edited line (GEd0) & genome edited first-generation lines (GEd1) of S. lycopersicum L. With these upshots, the study's key findings demonstrate outstanding value in developing sustainable multi-stress tolerance in S. lycopersicum L. and other crops to cope with climate change.
See https://www.frontiersin.org/articles/10.3389/fpls.2024.1304381/abstract
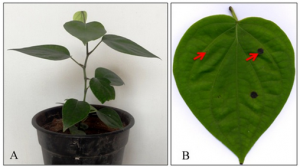
Figure: SlHyPRP1 and DEA1, the multiple stress responsive eight-cysteine motif family genes of tomato (Solanum lycopersicum L.) by B Saikia et al. 2020
|
|
|
|
[ Other News ]___________________________________________________
|


 Curently online :
Curently online :
 Total visitors :
Total visitors :
(243).png)