|
Conserved regulatory mechanism controls the development of cells with rooting functions in land plants
Wednesday, 2015/07/22 | 11:00:36
|
|
Thomas Ho Yuen Tam, Bruno Catarino, and Liam Dolan1 Department of Plant Sciences, University of Oxford, Oxford OX1 3RB, United Kingdom SignificanceThis work describes the discovery of an ancient genetic mechanism that was used to build rooting systems when plants colonized the relatively dry continental surfaces >470 million years ago. We demonstrate that a group of basic helix–loop–helix transcription factors—the LOTUS JAPONICUS ROOTHAIRLESS1-LIKE proteins—is part of a conserved auxin-regulated gene network that controls the development of tip-growing cells with rooting functions among extant land plants. This result suggests that this mechanism was active in the common ancestor of most land plants and facilitated the development of early land plant filamentous rooting systems, crucial for the successful colonization of the land by plants. AbstractLand plants develop filamentous cells—root hairs, rhizoids, and caulonemata—at the interface with the soil. Members of the group XI basic helix–loop–helix (bHLH) transcription factors encoded by LOTUS JAPONICUS ROOTHAIRLESS1-LIKE (LRL) genes positively regulate the development of root hairs in the angiosperms Lotus japonicus, Arabidopsis thaliana, and rice (Oryza sativa). Here we show that auxin promotes rhizoid and caulonema development by positively regulating the expression of PpLRL1 and PpLRL2, the two LRL genes in the Physcomitrella patens genome. Although the group VIII bHLH proteins, AtROOT HAIR DEFECTIVE6 and AtROOT HAIR DEFECTIVE SIX-LIKE1, promote root-hair development by positively regulating the expression of AtLRL3 in A. thaliana, LRL genes promote rhizoid development independently of PpROOT HAIR DEFECTIVE SIX-LIKE1 and PpROOT HAIR DEFECITVE SIX-LIKE2 (PpRSL1 and PpRSL2) gene function in P. patens. Together, these data demonstrate that both LRL and RSL genes are components of an ancient auxin-regulated gene network that controls the development of tip-growing cells with rooting functions among most extant land plants. Although this network has diverged in the moss and the angiosperm lineages, our data demonstrate that the core network acted in the last common ancestor of the mosses and angiosperms that existed sometime before 420 million years ago.
See: http://www.pnas.org/content/112/29/E3959.abstract.html?etoc PNAS uly 21, 2015 vol. 112 no. 29 E3959-E3968
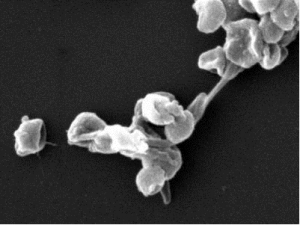
Fig. 5. Auxin-regulated caulonema and rhizoid development require PpLRL1 and PpLRL2 function. (A) Four-week-old protonema grown with 0 µM (−NAA) or 0.1 µM (+NAA) NAA. Auxin treatment induced the activity of the PpLRL1 and PpLRL2 promoters. [Scale bars: 5 mm (columns 1 and 4) or 500 µm (columns 2, 3, 5, and 6).] (B) The Pplrl1 Pplrl2 mutants are partially insensitive to auxin. (Scale bars: 5 mm.) (C) Close-up of a gametophore from 2-wk-old protonema grown with 0.1 µM NAA. Auxin failed to induce rhizoid development in the Pplrl1 Pplrl2 double mutants. (Scale bars: 500 µm.) |
|
|
|
[ Other News ]___________________________________________________
|


 Curently online :
Curently online :
 Total visitors :
Total visitors :
(17).png)