|
FLOURY ENDOSPERM 6 mutations enhance the sugary phenotype caused by the loss of ISOAMYLASE1 in barley
Wednesday, 2023/04/05 | 06:11:26
|
|
Ryo Matsushima, Hiroshi Hisano, Ivan Galis, Satoko Miura, Naoko Crofts, Yuto Takenaka, Naoko F. Oitome, Takeshi Ishimizu, Naoko Fujita & Kazuhiro Sato Theoretical and Applied Genetics April 2023; vol. 136, Article number: 94 Published: April 3 2023 Key messageBarley double mutants in two genes involved in starch granule morphology, HvFLO6 and HvISA1, had impaired starch accumulation and higher grain sugar levels than either single mutant. AbstractStarch is a biologically and commercially important glucose polymer synthesized by plants as semicrystalline starch granules (SGs). Because SG morphology affects starch properties, mutants with altered SG morphology may be useful in breeding crops with desirable starch properties, including potentially novel properties. In this study, we employed a simple screen for mutants with altered SG morphology in barley (Hordeum vulgare). We isolated mutants that formed compound SGs together with the normal simple SGs in the endosperm and found that they were allelic mutants of the starch biosynthesis genes ISOAMYLASE1 (HvISA1) and FLOURY ENDOSPERM 6 (HvFLO6), encoding starch debranching enzyme and CARBOHYDRATE-BINDING MODULE 48-containing protein, respectively. We generated the hvflo6 hvisa1 double mutant and showed that it had significantly reduced starch biosynthesis and developed shrunken grains. In contrast to starch, soluble α-glucan, phytoglycogen, and sugars accumulated to higher levels in the double mutant than in the single mutants. In addition, the double mutants showed defects in SG morphology in the endosperm and in the pollen. This novel genetic interaction suggests that hvflo6 acts as an enhancer of the sugary phenotype caused by hvisa1 mutation.
See https://link.springer.com/article/10.1007/s00122-023-04339-5
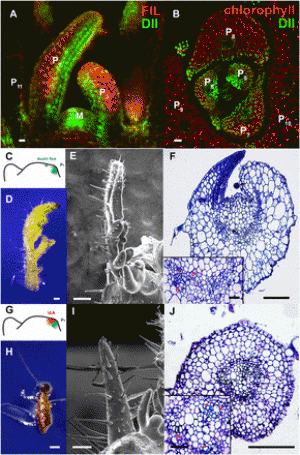
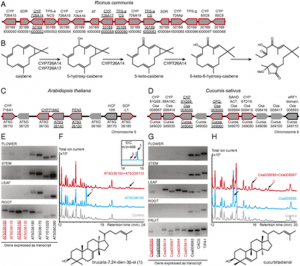
Figure 1: Isolation of new mutant alleles of HvISA1 and HvFLO6 in barley. a–c Mature grains of Haruna Nijo (a) and the new mutants hvisa1-3 (b) and hvflo6-2 (c). Front and side views are shown. Bars = 1 mm. d–i Iodine-stained thin sections of endosperm cells of Haruna Nijo (d), Morex (e), hvisa1-3 (f and g), and hvflo6-2 (h and i). Bars = 10 μm. j Structure of the HvISA1 gene on chr7H: 185,468,510..185,479,005 in MorexV3. The coding and untranslated regions are depicted as blue and white boxes, respectively. Introns are indicated by black lines. The exon–intron structure is based on the reported full-length cDNA (AB074189). The adenine in the translation start codon (ATG) is designated as + 1. hvisa1-3 has a base pair change from G to A at + 3387. This change introduces a TGA termination codon at the position of tryptophan (W)-490. The mutations in hvisa1-4 and hvisa1-5 are located at the splicing acceptor sites of the 12th and 14th introns, respectively. The reported sites of the Risø17 and Notch-2 mutations are indicated (Burton et al. 2002). k Immunoblot analysis using anti-HvISA1 antisera. The Ponceau S-stained membrane is shown. l Structure of the HvFLO6 gene on chr4H: 9,807,808..9,814,176 in MorexV3. The exon–intron structure is based on the reported full-length cDNA (AK373583). hvflo6-2 has two consecutive base pair changes at + 827 and + 828. The former change results in an amino acid substitution from arginine (R) to serine (S) at position 216. The latter change introduces a premature stop codon at glutamate (E)-217. The previously reported site of the Franubet (hvflo6-1) mutation is also indicated (Saito et al. 2018). The primer positions that were used for RT-PCR are indicated by black arrows
|
|
|
|
[ Other News ]___________________________________________________
|


 Curently online :
Curently online :
 Total visitors :
Total visitors :
(271).png)